Cavalloro, V.; Malacrida, A.; Miloso, M.; Ronchi, D.; Porta, A.; Fossati, A.; Gheza, G.; De Siervi, S.; Mantovani, S.; Oliviero, B.; Mondelli, M.U.; Pugliese, L.; Turato, C.; Martino, E.; Collina, S.
(2025) Biomedicine and Pharmacotherapy
DOI: 10.1016/j.biopha.2025.118208
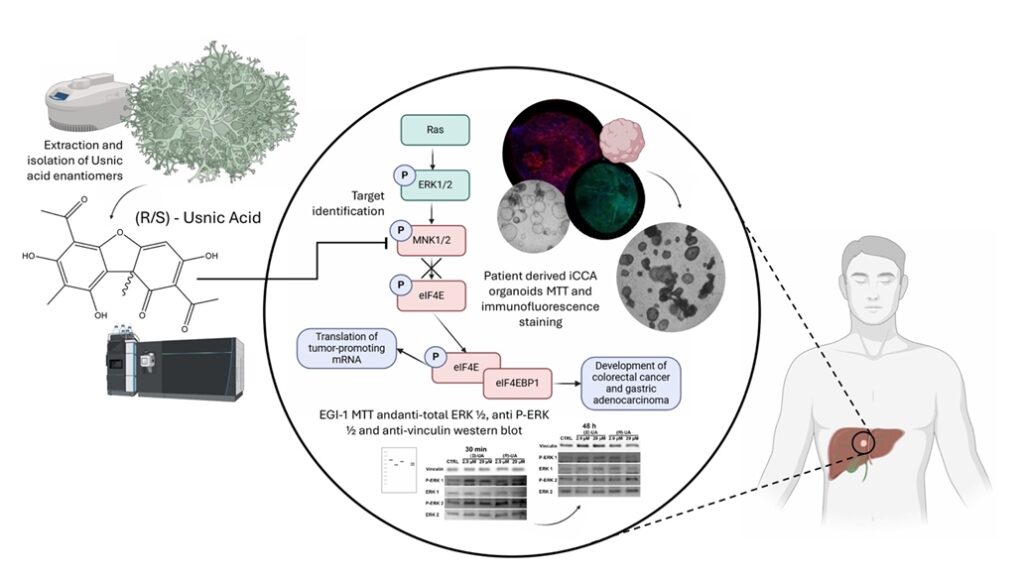
ABSTRACT: Cholangiocarcinoma (CC) remains one of the most challenging biliary tract malignancies, with limited therapeutic options and poor survival rates. We report the discovery and mechanistic investigation of usnic acid (UA) enantiomers as novel anti-CC agents. We identified the lichen Cladonia foliacea as a potential source of anticancer agents and developed a sustainable protocol to isolate (S)-UA as the most abundant metabolite. Our comprehensive comparative study of both enantiomers revealed time-dependent enantio-preference in their anti-cancer activity. While (S)-UA demonstrated twice the potency at 24 h, (R)-UA exhibited nearly ten-fold greater activity at 48 h and 72 h, particularly at lower concentrations (2.9 and 29 mM). Overall, both enantiomers inhibited EGI-1 cell proliferation in the micromolar range in a dose- and time-dependent manner. In silico studies and kinase profiling identified MNK2 as a primary target, with subsequent validation confirming direct binding and inhibition. Mechanistic studies demonstrated that UA enantiomers modulate the MAPK pathway, leading to decreased phosphorylation of eIF4E and suppression of cancer-promoting proteins. The successful translation of activity from 2D cell cultures to patient-derived 3D organoid models further validates their therapeutic potential. Our findings establish usnic acid as a promising natural product scaffold for CC treatment and provide detailed insights into its mechanism of action through MNK2 targeting and MAPK pathway modulation, with important considerations for enantiomer-specific temporal efficacy.
